27 June 2023
Elements, Isotopes, and Radioactive Decay
To kick off the AIRIE Blog series, it is back to basics with a refresher on elements, isotopes, and radioactive decay. With a solid understanding of these topics, we will be able to dig deep into rhenium-osmium (Re-Os) analysis and the nitty gritty details on how AIRIE produces geochronological data.
Rhenium and osmium are two of (roughly) 91 naturally occurring elements found in nature. Rhenium, atomic number 75 and the last stable element ever isolated, was found in 1925 by German scientists Walter Noddack, Ida Tacke-Noddack, and Otto Berg although Masataka Ogawa found it in Sri Lankan thorianite but misinterpreted it as, what would become, technetium. The name rhenium is from the Greek word "Rhenus" meaning the river "Rhine". Osmium, atomic number 76, was identified in 1803 in England by Smithson Tennant after dissolving a platinum alloy and noticing a residual solid (of osmium and iridium). The name osmium is from the Greek word "osme" meaning "smell" as Tennant noticed the strong odor produced by the residual solid.
Recall, isotopes of an element represent atomic configurations in which the number of electrons and protons is fixed, but the nucleus contains differing number of neutrons. Rhenium, atomic mass 186.207 with 75 electrons and protons, is comprised of two natural isotopes, 185Re (110 neutrons) and 187Re (112 neutrons), of which 187Re is radioactive. Osmium, atomic mass 190.23 with 76 electrons and protons, has seven naturally occurring isotopes; 184Os, 186Os, 187Os (daughter product of 187Re decay), 188Os, 189Os, 190Os, and 192Os. 186Os radioactively decays to 186Pt. Isotopes, and radioactive decay, are they cornerstones of geochronology and Re-Os analysis.
Radioactive decay is the process by which a radioactive atom emits radiation in the form of energy or particles in order to reach a more stable state. The three main types of decay are alpha, beta, and gamma. While the process is random at the scale of the individual atom, with a sufficiently large number of atoms, the rate of decay can be determined. This is represented by either the decay constant (lambda, l) or the half-life (T1/2) with the relationship T1/2 = ln(2) / l.
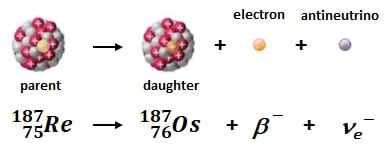
187Re decays to 187Os by beta decay in which a neutron is converted into a proton resulting emission of an electron and an antineutrino. The newly formed proton increases the atom's atomic number by 1 (75 --> 76) without a change in nuclear mass, hence the formation of 187Os. T1/2 is 41.6 billion years (Ga; l = 1.666x10-11). With a half-life roughly 10x the age of the earth, the Re-Os geochronometer can date all but the youngest geologic samples; the youngest sample analyzed at AIRIE is ~1.1 million years old (Ma) while the oldest is ~3.3 Ga (a range representing 72% of Earth's history).